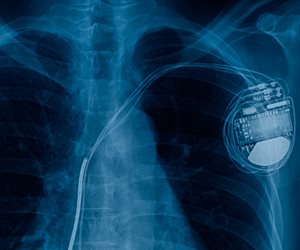
Over the last few years, some patients have been receiving implantable cardioverter-defibrillators (ICDs) that are subject to Class I recalls. These devices have continued to be implanted, breaking with precedent, largely based on assurances and safety analyses from the device manufacturer, according to a perspective article published in the New England Journal of Medicine.
“The decision to continue to market and implant the relevant devices relied primarily on the manufacturer’s analysis of its own data, which aren’t widely available. Patients and the medical community are therefore confronted with an important clinical decision, without independent science to guide them,” wrote Daniel B. Kramer, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and Robert G. Hauser, of the Minneapolis Heart Institute Foundation…