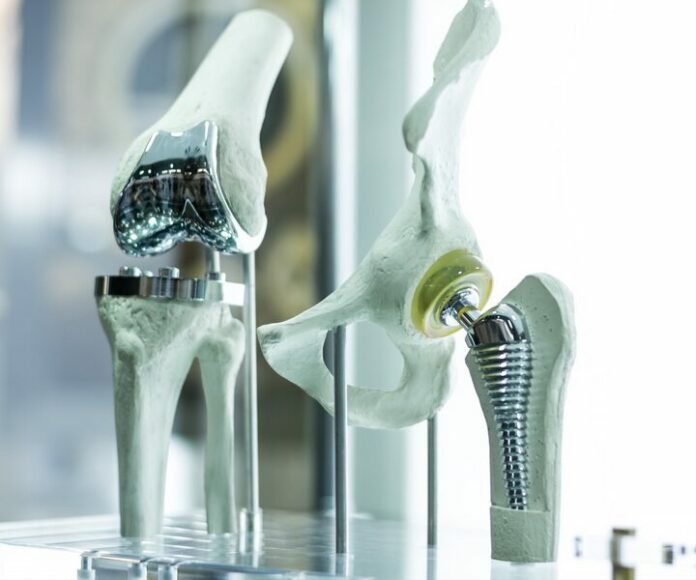
Medical device makers are asking the US Food and Drug Administration (FDA) to make exceptions to its recently proposed guidance on metallic and calcium phosphate coatings on orthopedic devices. They also asked the agency to address the utility of other guidances that may be affected if the proposed guidance is finalized.
On 22 January, the FDA published a draft guidance entitled « Characterization of Metallic Coatings and/or Calcium Phosphate Coatings on Orthopedic Devices. » The docket for the guidance received seven comments before it closed. (RELATED: FDA proposes guidance on orthopedic product coatings, Regulatory Focus 22 January 2024)
The medtech lobby group AdvaMed submitted extensive comments that included requests for several clarifications to the guidance, including whether FDA thinks additive manufactured porous structures in the form of coatings or bulk porous structures should be a part of the guidance…